Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.
Topic Contents
- General Information About Childhood Central Nervous System (CNS) Atypical Teratoid / Rhabdoid Tumor
- Tumor Biology of Childhood CNS Atypical Teratoid / Rhabdoid Tumor
- Stage Information for Childhood CNS Atypical Teratoid / Rhabdoid Tumor
- Treatment of Childhood CNS Atypical Teratoid / Rhabdoid Tumor
- Treatment of Recurrent Childhood CNS Atypical Teratoid / Rhabdoid Tumor
- Current Clinical Trials
- Latest Updates to This Summary (04 / 05 / 2024)
- About This PDQ Summary
Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment (PDQ®): Treatment - Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Childhood Central Nervous System (CNS) Atypical Teratoid / Rhabdoid Tumor
Primary brain tumors, including atypical teratoid/rhabdoid tumors (AT/RTs), are a diverse group of diseases that together constitute the most common solid tumors of childhood. The PDQ childhood brain tumor treatment summaries are primarily organized according to the World Health Organization classification of nervous system tumors.[1,2] Brain tumors are classified according to histology, but immunohistochemical analysis, cytogenetic and molecular genetic findings, and measures of mitotic activity are increasingly used in tumor diagnosis and classification. Tumor location, extent of spread, and age at diagnosis are important factors that affect treatment and prognosis.[3,4,5] For a description of the classification of nervous system tumors and a link to the corresponding treatment summary for each type of brain tumor, see Childhood Brain and Spinal Cord Tumors Summary Index.
CNS AT/RT is a rare, clinically aggressive tumor that most often affects children aged 3 years and younger but can occur in older children and adults. Approximately one-half of AT/RTs arise in the posterior fossa.[6] The diagnostic evaluation includes magnetic resonance imaging (MRI) of the neuraxis and lumbar cerebrospinal fluid examination. AT/RT has been linked to somatic and germline variants of SMARCB1 and, less commonly, SMARCA4, both of which act as tumor suppressor genes.[7] There is no evidence-based standard treatment for children with AT/RT. Multimodality treatment consisting of surgery, chemotherapy (including high-dose chemotherapy), and radiation therapy is under evaluation in controlled clinical trials.
Based on current biological understanding, AT/RT is part of a larger family of rhabdoid tumors. In this summary, the term AT/RT refers to CNS tumors only, and the term rhabdoid tumor reflects the possibility of both CNS and non-CNS tumors. Unless specifically noted in the text, this summary refers to CNS AT/RT.
Childhood and adolescent cancer survivors require close monitoring because side effects of cancer therapy may persist or develop months or years after treatment. For specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors, see Late Effects of Treatment for Childhood Cancer.
Incidence
The exact incidence of childhood CNS AT/RT is difficult to determine because the tumor is rare and has only been recognized since 1996.[8]
- In two prospective studies performed by the Children's Cancer Group and the Pediatric Oncology Group in North America, retrospective review disclosed that approximately 10% of children aged 3 years or younger at diagnosis with brain tumors had AT/RTs.[9]
- A study completed in Taiwan found that AT/RTs account for 26% of primitive or embryonal tumors in children younger than 3 years.[10]
- The Austrian Brain Tumor Registry (recruitment period, 1996–2006) confirmed that AT/RTs represented the sixth most common malignant brain tumor among 311 newly diagnosed children (6.1%), with a peak incidence during the first 2 years of life.[11]
The incidence in older patients is unknown. However, in the Central Nervous System Atypical Teratoid/Rhabdoid Tumor Registry (AT/RT Registry), 12 of the 42 patients (29%) were older than 36 months at the time of diagnosis.[12]
Anatomy
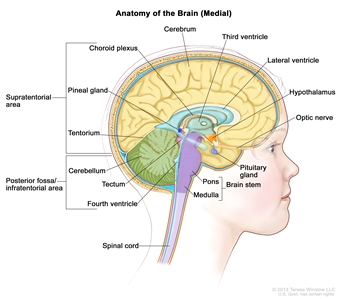
Anatomy of the inside of the brain, showing the pineal and pituitary glands, optic nerve, ventricles (with cerebrospinal fluid shown in blue), and other parts of the brain. The tentorium separates the cerebrum from the cerebellum. The infratentorium (posterior fossa) is the region below the tentorium that contains the brain stem, cerebellum, and fourth ventricle. The supratentorium is the region above the tentorium and denotes the region that contains the cerebrum.
Clinical Presentation
Childhood AT/RT is a clinically aggressive tumor that primarily occurs in children younger than 3 years, but it also can occur in older children and adults.[13,14]
Approximately one-half of all AT/RTs arise in the posterior fossa, although they can occur anywhere in the CNS.[6,9] Tumors of the posterior fossa may occur in the cerebellopontine angle or more midline. Involvement of individual cranial nerves has been noted.[15]
Because AT/RTs grow rapidly, patients often have a fairly short history of progressive symptoms, measured in days to weeks. Signs and symptoms depend on tumor location. Young patients with posterior fossa tumors usually present with symptoms related to hydrocephalus, which include the following:
- Early-morning headaches.
- Vomiting.
- Lethargy.
- Increased head circumference.
They may also develop ataxia, regression of motor skills, or localizing symptoms related to cranial nerve dysfunction.
Registry data suggest that 25% to 30% of patients present with disseminated disease.[5,12,16] Dissemination is typically through leptomeningeal pathways seeding the spine and other areas of the brain. Up to 35% of patients present with germline variants and may be prone to synchronous, multifocal tumors.[17,18,19,20]
Diagnostic Evaluation
All patients with suspected AT/RT should undergo MRI of the brain and spine. Unless medically contraindicated, the lumbar cerebrospinal fluid should be inspected for evidence of tumor. Patients may also undergo renal ultrasonography to detect synchronous tumors. Germline testing is also indicated.
AT/RTs cannot be reliably distinguished from other malignant brain tumors on the basis of clinical history or radiographic evaluation alone. Surgery is necessary to obtain tissue and confirm the diagnosis. Immunohistochemical staining for loss of SMARCB1 protein expression is also used to confirm the diagnosis.[21,22] Methylation array analysis has become an important adjunct to confirm the AT/RT subtype.[3,4]
Prognosis
Prognostic factors that affect survival for patients with AT/RTs are not fully delineated.
Known factors associated with a poor outcome include the following:
- Germline variant.[23]
- Younger age, especially younger than 1 year.[5,24]
- Metastases at diagnosis.[24]
- Subtotal resection.[25]
- Specific AT/RT molecular subtypes.[3,5,26]
Most published data on outcomes of patients with AT/RT are from small series and are retrospective in nature. Initial retrospective studies reported an average survival from diagnosis of only about 12 months.[8,9,13,25,27] In a retrospective report, 2-year overall survival (OS) was better for patients who underwent a gross-total resection than for those who had a subtotal resection. However, in this study, the effect of radiation therapy on survival was less clear.[25]
There are reports of long-term survivors.[28] Notably, improved survival has been reported for those who received intensive multimodality therapy.[16,19]
- Children aged 3 years and older with AT/RT who received postoperative craniospinal irradiation and high-dose, alkylator-based chemotherapy had improved survival compared with patients younger than 3 years. In this report, the incidence of leptomeningeal metastases was also higher in the infant patients.[29]
- In one prospective study of 25 children with AT/RT who received intensive multimodality therapy, including radiation and intrathecal chemotherapy, the reported 2-year progression-free survival rate was 53%, and the OS rate was 70%.[30]
- For patients in the prospective ACNS0333 (NCT00653068) trial, the 4-year event-free survival rate was 37%, and the 4-year OS rate was 43%.[31]
- In the prospective European Rhabdoid Registry series, patients aged 1 year and older with an AT/RT tyrosinase (TYR) subgroup designation demonstrated a 5-year OS rate of 71%, while those younger than 1 year with a non-TYR AT/RT had a very poor survival rate.[5] These data were confirmed in two other trials.[26]
References:
- Louis DN, Perry A, Wesseling P, et al.: The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23 (8): 1231-1251, 2021.
- WHO Classification of Tumours Editorial Board, ed.: WHO Classification of Tumours: Central Nervous System Tumours. Vol. 6. 5th ed. IARC Press; 2021.
- Federico A, Thomas C, Miskiewicz K, et al.: ATRT-SHH comprises three molecular subgroups with characteristic clinical and histopathological features and prognostic significance. Acta Neuropathol 143 (6): 697-711, 2022.
- Lu VM, Di L, Eichberg DG, et al.: Age of diagnosis clinically differentiates atypical teratoid/rhabdoid tumors diagnosed below age of 3 years: a database study. Childs Nerv Syst 37 (4): 1077-1085, 2021.
- Frühwald MC, Hasselblatt M, Nemes K, et al.: Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors. Neuro Oncol 22 (7): 1006-1017, 2020.
- Dho YS, Kim SK, Cheon JE, et al.: Investigation of the location of atypical teratoid/rhabdoid tumor. Childs Nerv Syst 31 (8): 1305-11, 2015.
- Hasselblatt M, Nagel I, Oyen F, et al.: SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol 128 (3): 453-6, 2014.
- Rorke LB, Packer RJ, Biegel JA: Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 85 (1): 56-65, 1996.
- Packer RJ, Biegel JA, Blaney S, et al.: Atypical teratoid/rhabdoid tumor of the central nervous system: report on workshop. J Pediatr Hematol Oncol 24 (5): 337-42, 2002 Jun-Jul.
- Ho DM, Hsu CY, Wong TT, et al.: Atypical teratoid/rhabdoid tumor of the central nervous system: a comparative study with primitive neuroectodermal tumor/medulloblastoma. Acta Neuropathol 99 (5): 482-8, 2000.
- Woehrer A, Slavc I, Waldhoer T, et al.: Incidence of atypical teratoid/rhabdoid tumors in children: a population-based study by the Austrian Brain Tumor Registry, 1996-2006. Cancer 116 (24): 5725-32, 2010.
- Hilden JM, Meerbaum S, Burger P, et al.: Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol 22 (14): 2877-84, 2004.
- Burger PC, Yu IT, Tihan T, et al.: Atypical teratoid/rhabdoid tumor of the central nervous system: a highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: a Pediatric Oncology Group study. Am J Surg Pathol 22 (9): 1083-92, 1998.
- Lutterbach J, Liegibel J, Koch D, et al.: Atypical teratoid/rhabdoid tumors in adult patients: case report and review of the literature. J Neurooncol 52 (1): 49-56, 2001.
- Lobón-Iglesias MJ, Andrianteranagna M, Han ZY, et al.: Imaging and multi-omics datasets converge to define different neural progenitor origins for ATRT-SHH subgroups. Nat Commun 14 (1): 6669, 2023.
- Bartelheim K, Nemes K, Seeringer A, et al.: Improved 6-year overall survival in AT/RT - results of the registry study Rhabdoid 2007. Cancer Med 5 (8): 1765-75, 2016.
- Biegel JA, Fogelgren B, Wainwright LM, et al.: Germline INI1 mutation in a patient with a central nervous system atypical teratoid tumor and renal rhabdoid tumor. Genes Chromosomes Cancer 28 (1): 31-7, 2000.
- Bourdeaut F, Lequin D, Brugières L, et al.: Frequent hSNF5/INI1 germline mutations in patients with rhabdoid tumor. Clin Cancer Res 17 (1): 31-8, 2011.
- Seeringer A, Reinhard H, Hasselblatt M, et al.: Synchronous congenital malignant rhabdoid tumor of the orbit and atypical teratoid/rhabdoid tumor--feasibility and efficacy of multimodal therapy in a long-term survivor. Cancer Genet 207 (9): 429-33, 2014.
- Nemes K, Clément N, Kachanov D, et al.: The extraordinary challenge of treating patients with congenital rhabdoid tumors-a collaborative European effort. Pediatr Blood Cancer 65 (6): e26999, 2018.
- Bruggers CS, Bleyl SB, Pysher T, et al.: Clinicopathologic comparison of familial versus sporadic atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system. Pediatr Blood Cancer 56 (7): 1026-31, 2011.
- Margol AS, Judkins AR: Pathology and diagnosis of SMARCB1-deficient tumors. Cancer Genet 207 (9): 358-64, 2014.
- Kordes U, Gesk S, Frühwald MC, et al.: Clinical and molecular features in patients with atypical teratoid rhabdoid tumor or malignant rhabdoid tumor. Genes Chromosomes Cancer 49 (2): 176-81, 2010.
- Dufour C, Beaugrand A, Le Deley MC, et al.: Clinicopathologic prognostic factors in childhood atypical teratoid and rhabdoid tumor of the central nervous system: a multicenter study. Cancer 118 (15): 3812-21, 2012.
- Lafay-Cousin L, Hawkins C, Carret AS, et al.: Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer 48 (3): 353-9, 2012.
- Upadhyaya SA, Robinson GW, Onar-Thomas A, et al.: Relevance of Molecular Groups in Children with Newly Diagnosed Atypical Teratoid Rhabdoid Tumor: Results from Prospective St. Jude Multi-institutional Trials. Clin Cancer Res 27 (10): 2879-2889, 2021.
- Athale UH, Duckworth J, Odame I, et al.: Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol 31 (9): 651-63, 2009.
- Olson TA, Bayar E, Kosnik E, et al.: Successful treatment of disseminated central nervous system malignant rhabdoid tumor. J Pediatr Hematol Oncol 17 (1): 71-5, 1995.
- Tekautz TM, Fuller CE, Blaney S, et al.: Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol 23 (7): 1491-9, 2005.
- Chi SN, Zimmerman MA, Yao X, et al.: Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 27 (3): 385-9, 2009.
- Reddy AT, Strother DR, Judkins AR, et al.: Efficacy of High-Dose Chemotherapy and Three-Dimensional Conformal Radiation for Atypical Teratoid/Rhabdoid Tumor: A Report From the Children's Oncology Group Trial ACNS0333. J Clin Oncol 38 (11): 1175-1185, 2020.
Tumor Biology of Childhood CNS Atypical Teratoid / Rhabdoid Tumor
Childhood central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) was first described as a discrete clinical entity in 1987 [1] based on its distinctive pathological and genetic characteristics. Before then, it was most often classified as a medulloblastoma, CNS primitive neuroectodermal tumor (CNS PNET), or choroid plexus carcinoma. The World Health Organization (WHO) classifies AT/RT as an embryonal grade IV neoplasm.[2]
Histologically, AT/RT is morphologically heterogeneous, typically containing sheets of large epithelioid cells with abundant eosinophilic cytoplasm and scattered rhabdoid cells, most often with accompanying components of primitive neuroectodermal cells (small round blue cells), mesenchymal cells, and/or glial cells.[3]
Immunohistochemical staining for epithelial markers (cytokeratin or epithelial membrane antigen), glial fibrillary acidic protein, synaptophysin (or neurofilament), and smooth muscle (desmin) may help to identify the heterogeneity of differentiation, but will vary depending on the cellular composition.[4] Rhabdoid cells, while not present in all AT/RTs, will express vimentin, epithelial membrane antigen, and smooth muscle actin.
Immunohistochemical staining for the SMARCB1 protein is useful in establishing the diagnosis of AT/RT. A loss of SMARCB1 staining is noted in neoplastic cells, but staining is retained in non-neoplastic cells (e.g., vascular endothelial cells).[5,6,7]
AT/RT is a rapidly growing tumor that can have an MIB-1 labeling index of 50% to 100%.[8]
Genomics of CNS Atypical Teratoid/Rhabdoid Tumor (AT/RT)
SMARCB1andSMARCA4genes
AT/RT was the first primary pediatric brain tumor in which a candidate tumor suppressor gene, SMARCB1, was identified.[9]SMARCB1 is genomically altered in most rhabdoid tumors, including CNS, renal, and extrarenal rhabdoid malignancies.[9]SMARCB1 is a component of the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin-remodeling complex.[10]
Rare cases of rhabdoid tumors expressing SMARCB1 and lacking SMARCB1 variants have also been associated with somatic or germline variants of SMARCA4, another member of the SWI/SNF chromatin-remodeling complex.[7,11,12]
Less commonly, SMARCA4-negative (with retained SMARCB1) tumors have been described.[7,11,12] Loss of SMARCB1 or SMARCA4 staining is a defining marker for AT/RT.
The 2021 WHO classification defines AT/RT by the presence of either SMARCB1 or SMARCA4 alterations. Tumors with histological features of AT/RT that lack these genomic alterations are termed CNS embryonal tumors with rhabdoid features.[13]
Despite the absence of recurring genomic alterations beyond SMARCB1 and SMARCA4,[14,15,16] biologically, relatively distinctive subsets of AT/RT have been identified.[17,18,19] In one study, three distinctive subsets of AT/RT were identified through the use of DNA methylation arrays for 150 AT/RT tumors and gene expression arrays for 67 AT/RT tumors:[18]
- AT/RT tyrosinase (TYR): This subset represented approximately one-third of cases and was characterized by elevated expression of melanosomal markers such as TYR (the gene encoding tyrosinase). Cases in this subset were primarily infratentorial, with most presenting in children aged 0 to 1 year and showing chromosome 22q loss.[18] For patients with AT/RT TYR, the mean overall survival (OS) was 37 months in a clinically heterogeneous group (95% confidence interval [CI], 18–56 months).[20] In the prospective European Rhabdoid Registry (EU-RHAB) series, patients aged 1 year and older with AT/RT TYR demonstrated a 5-year OS rate of 71%, while those younger than 1 year with a non-TYR AT/RT had a very poor survival rate.[21]
- AT/RT sonic hedgehog (SHH): This subset represented approximately 40% of cases and was characterized by elevated expression of genes in the SHH pathway (e.g., GLI2 and MYCN). Cases in this subset occurred with similar frequency in the supratentorium and infratentorium. While most patients presented before the age of 2 years, approximately one-third of patients presented between the ages of 2 and 5 years.[18] For patients with AT/RT SHH, the mean OS was 16 months (95% CI, 8–25 months).[20]
In a subsequent study, the AT/RT SHH subgroup was further divided into three subtypes: SHH-1A, SHH-1B, and SHH-2.[22] Children older than 3 years who harbored the SHH-1B signature experienced the most favorable outcomes.
- AT/RT MYC: This subset represented approximately one-fourth of cases and was characterized by elevated expression of MYC. AT/RT MYC cases tended to occur in the supratentorial compartment. While most AT/RT MYC cases occurred by the age of 5 years, AT/RT MYC represented the most common subset diagnosed at age 6 years and older. Focal deletions of SMARCB1 were the most common mechanism of SMARCB1 loss for this subset.[18] For patients with AT/RT MYC, the mean OS was 13 months (95% CI, 5–22 months).[20]
Loss of SMARCB1 or SMARCA4 protein expression has therapeutic significance, because this loss creates a dependence of the cancer cells on EZH2 activity.[23] Preclinical studies have shown that some AT/RT xenograft lines with SMARCB1 loss respond to EZH2 inhibitors with tumor growth inhibition and occasional tumor regression.[24,25] In a study of the EZH2 inhibitor tazemetostat, objective responses were observed in adult patients whose tumors had either SMARCB1 or SMARCA4 loss (non-CNS malignant rhabdoid tumors and epithelioid sarcoma).[26] For more information, see the Treatment of Recurrent Childhood CNS Atypical Teratoid/Rhabdoid Tumor section.
Cribriform Neuroepithelial Tumor
Cribriform neuroepithelial tumor has genomic and epigenomic characteristics that are very similar to those of AT/RT TYR.[20] The 2021 WHO Classification lists cribriform neuroepithelial tumor as a provisional entity. Like AT/RT, cribriform neuroepithelial tumor occurs in young children (median age, 1–2 years) and tumor cells lack SMARCB1 expression. Histologically, cribriform neuroepithelial tumor is characterized by the presence of cribriform strands and ribbons, but there is an absence of rhabdoid tumor cells with abundant eosinophilic cytoplasm. Like AT/RT TYR, tyrosinase expression is commonly observed. The outcome of patients with cribriform neuroepithelial tumor is more favorable than the outcome of patients with AT/RT TYR. In one study, only one death was reported among ten children with cribriform neuroepithelial tumor.[20]
Rhabdoid Tumor Predisposition Syndrome (RTPS)
RTPS, which is primarily related to germline SMARCB1 alterations (and less commonly to germline SMARCA4 alterations), has been clearly defined.[9,27] RTPS caused by SMARCB1 germline alterations is termed RTPS Type 1, while RTPS due to a SMARCA4 germline variant is called RTPS Type 2. RTPS is highly suggested in patients with synchronous occurrence of extracranial malignant rhabdoid tumor (kidney or soft tissue) and AT/RT, bilateral malignant rhabdoid tumors of the kidney, or malignant rhabdoid tumors in two or more siblings.
This syndrome is manifested by a marked predisposition to the development of malignant rhabdoid tumors in infancy and early childhood. Up to one-third of AT/RTs are thought to arise in the setting of RTPS, and most of these occur within the first year of life. The most common non-CNS malignancy of RTPS is malignant rhabdoid tumor of the kidney, which is also noted in infancy.[28,29]
A study of 65 children with rhabdoid tumors found that 23 (35%) had germline variants and/or deletions of SMARCB1.[5] Children with germline alterations in SMARCB1 presented at an earlier age than did sporadic cases (median age, approximately 5 months vs. 18 months) and were more likely to present with synchronous, multifocal tumors.[5] One parent was found to be a carrier of the SMARCB1 germline abnormality in 7 of 22 evaluated cases showing germline alterations, with four of the carrier parents being unaffected by SMARCB1-associated cancers.[5] This finding indicates that AT/RT shows an autosomal dominant inheritance pattern with incomplete penetrance.
Gonadal mosaicism has also been observed, as evidenced by families in which multiple siblings are affected by AT/RT and have identical SMARCB1 alterations, but both parents lack a SMARCB1 variant/deletion.[5,6] Screening for germline SMARCB1 variants in children diagnosed with AT/RT is suggested for counseling families on the genetic implications of their child's AT/RT diagnosis.[5] Preliminary recommendations for the genetic evaluation and subsequent presymptomatic screening of nonaffected variant carriers (including parents and siblings of affected patients) have been reported and are likely to evolve as the understanding of RTPS improves.[28,29,30] In patients with a predisposition to AT/RT, whole-body magnetic resonance imaging may help to identify synchronous rhabdoid tumors outside of the CNS.
For more information about RTPS1 and SMARCB1, see Rhabdoid Tumor Predisposition Syndrome Type 1.
References:
- Rorke LB, Packer RJ, Biegel JA: Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 85 (1): 56-65, 1996.
- Oztek MA, Noda SM, Romberg EK, et al.: Changes to pediatric brain tumors in 2021 World Health Organization classification of tumors of the central nervous system. Pediatr Radiol 53 (3): 523-543, 2023.
- Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. IARC Press, 2016.
- McLendon RE, Adekunle A, Rajaram V, et al.: Embryonal central nervous system neoplasms arising in infants and young children: a pediatric brain tumor consortium study. Arch Pathol Lab Med 135 (8): 984-93, 2011.
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011.
- Bruggers CS, Bleyl SB, Pysher T, et al.: Clinicopathologic comparison of familial versus sporadic atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system. Pediatr Blood Cancer 56 (7): 1026-31, 2011.
- Hasselblatt M, Gesk S, Oyen F, et al.: Nonsense mutation and inactivation of SMARCA4 (BRG1) in an atypical teratoid/rhabdoid tumor showing retained SMARCB1 (INI1) expression. Am J Surg Pathol 35 (6): 933-5, 2011.
- Kleihues P, Louis DN, Scheithauer BW, et al.: The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61 (3): 215-25; discussion 226-9, 2002.
- Biegel JA, Tan L, Zhang F, et al.: Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res 8 (11): 3461-7, 2002.
- Biegel JA, Kalpana G, Knudsen ES, et al.: The role of INI1 and the SWI/SNF complex in the development of rhabdoid tumors: meeting summary from the workshop on childhood atypical teratoid/rhabdoid tumors. Cancer Res 62 (1): 323-8, 2002.
- Schneppenheim R, Frühwald MC, Gesk S, et al.: Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet 86 (2): 279-84, 2010.
- Hasselblatt M, Nagel I, Oyen F, et al.: SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol 128 (3): 453-6, 2014.
- WHO Classification of Tumours Editorial Board, ed.: WHO Classification of Tumours: Central Nervous System Tumours. Vol. 6. 5th ed. IARC Press; 2021.
- Lee RS, Stewart C, Carter SL, et al.: A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest 122 (8): 2983-8, 2012.
- Kieran MW, Roberts CW, Chi SN, et al.: Absence of oncogenic canonical pathway mutations in aggressive pediatric rhabdoid tumors. Pediatr Blood Cancer 59 (7): 1155-7, 2012.
- Hasselblatt M, Isken S, Linge A, et al.: High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer 52 (2): 185-90, 2013.
- Torchia J, Picard D, Lafay-Cousin L, et al.: Molecular subgroups of atypical teratoid rhabdoid tumours in children: an integrated genomic and clinicopathological analysis. Lancet Oncol 16 (5): 569-82, 2015.
- Johann PD, Erkek S, Zapatka M, et al.: Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell 29 (3): 379-93, 2016.
- Upadhyaya SA, Robinson GW, Onar-Thomas A, et al.: Relevance of Molecular Groups in Children with Newly Diagnosed Atypical Teratoid Rhabdoid Tumor: Results from Prospective St. Jude Multi-institutional Trials. Clin Cancer Res 27 (10): 2879-2889, 2021.
- Johann PD, Hovestadt V, Thomas C, et al.: Cribriform neuroepithelial tumor: molecular characterization of a SMARCB1-deficient non-rhabdoid tumor with favorable long-term outcome. Brain Pathol 27 (4): 411-418, 2017.
- Frühwald MC, Hasselblatt M, Nemes K, et al.: Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors. Neuro Oncol 22 (7): 1006-1017, 2020.
- Federico A, Thomas C, Miskiewicz K, et al.: ATRT-SHH comprises three molecular subgroups with characteristic clinical and histopathological features and prognostic significance. Acta Neuropathol 143 (6): 697-711, 2022.
- Wilson BG, Wang X, Shen X, et al.: Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 18 (4): 316-28, 2010.
- Knutson SK, Warholic NM, Wigle TJ, et al.: Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A 110 (19): 7922-7, 2013.
- Kurmasheva RT, Sammons M, Favours E, et al.: Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 64 (3): , 2017.
- Italiano A, Soria JC, Toulmonde M, et al.: Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19 (5): 649-659, 2018.
- Biegel JA, Fogelgren B, Wainwright LM, et al.: Germline INI1 mutation in a patient with a central nervous system atypical teratoid tumor and renal rhabdoid tumor. Genes Chromosomes Cancer 28 (1): 31-7, 2000.
- Frühwald MC, Nemes K, Boztug H, et al.: Current recommendations for clinical surveillance and genetic testing in rhabdoid tumor predisposition: a report from the SIOPE Host Genome Working Group. Fam Cancer 20 (4): 305-316, 2021.
- Nemes K, Bens S, Bourdeaut F, et al.: Rhabdoid Tumor Predisposition Syndrome. In: Adam MP, Feldman J, Mirzaa GM, et al., eds.: GeneReviews. University of Washington, Seattle, 1993-2024, pp. Available online. Last accessed December 15, 2023.
- Foulkes WD, Kamihara J, Evans DGR, et al.: Cancer Surveillance in Gorlin Syndrome and Rhabdoid Tumor Predisposition Syndrome. Clin Cancer Res 23 (12): e62-e67, 2017.
Stage Information for Childhood CNS Atypical Teratoid / Rhabdoid Tumor
There is no evidence-based staging system for childhood central nervous system atypical teratoid/rhabdoid tumor. For treatment purposes, patients are classified as having newly diagnosed or recurrent disease, with or without neuraxis dissemination.
Treatment of Childhood CNS Atypical Teratoid / Rhabdoid Tumor
An evidence-based standard treatment for children with newly diagnosed central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) has not yet been defined. Given the highly aggressive nature of the tumor, most patients have been treated with intensive multimodality therapy. However, the extent of treatment, particularly for radiation therapy, is limited because of the young age of most patients.
Treatment options for newly diagnosed CNS AT/RT include the following:
- Surgery, chemotherapy, and radiation therapy (multimodality therapy).
Surgery, Chemotherapy, and Radiation Therapy (Multimodality Therapy)
The extent of surgical resection may affect survival. Data from the Central Nervous System Atypical Teratoid/Rhabdoid Tumor Registry (AT/RT Registry) suggest that patients who have had a complete resection may have a longer median survival. However, complete surgical resection is often difficult because of the invasive nature of the tumor.[1]
Chemotherapy has been the main adjuvant therapy for very young children with AT/RT. Cooperative group studies that included children younger than 36 months demonstrated poor survival with standard chemotherapeutic regimens alone.[2] The Children's Cancer Group reported a 2-year event-free survival (EFS) rate of 14% for 28 children younger than 36 months who were treated with multiagent chemotherapy.[3]
Intensive regimens that use varying combinations of high-dose chemotherapy,[4][Level of evidence C1]; [5,6][Level of evidence C2] intrathecal chemotherapy, and radiation therapy have led to prolonged survival for some patients.
Only two prospective trials for children with CNS AT/RT have been completed. In an institutional prospective trial, children were treated with a modified Intergroup Rhabdomyosarcoma Study-III (IRS-III) protocol, using intrathecal chemotherapy and radiation therapy. Of the subset of 20 children who completed therapy, the 2-year progression-free survival (PFS) rate was 53%, and the overall survival (OS) rate was 70%. Survival was better for patients who had a complete resection.[7][Level of evidence C1] In the Children's Oncology Group (COG) ACNS0333 (NCT00653068) study, patients were treated with intensive induction chemotherapy, followed by high-dose chemotherapy with autologous stem cell rescue and radiation therapy. The 4-year PFS rate was 37%, and the OS rate was 43%.[8][Level of evidence B4]
Thirteen patients in the AT/RT Registry were treated with high-dose chemotherapy with hematopoietic stem cell rescue as part of initial therapy.[1] Four of these patients, two of whom also received radiation, were alive without progressive disease 21.5 to 90 months after diagnosis at last report. Of 15 evaluable children (all younger than 32 months at diagnosis) who were on a chemotherapy Head Start III protocol, 2 survived for more than 47 months.[9][Level of evidence C1]
Radiation therapy appears to have a positive impact on survival for patients with AT/RT.[10]
Evidence (radiation therapy):
- Of the 42 patients in the AT/RT Registry, 13 (31%) received radiation therapy in addition to chemotherapy as part of their primary therapy.[1] The radiation field was to the primary tumor bed in nine children, and the radiation field was to the tumor bed and the craniospinal axis in four children.
- The median survival of these patients was 48 months, compared with 16.75 months for all patients in the registry.
- In a retrospective series of 31 patients with AT/RT from the St. Jude Children's Research Hospital, the following results were reported:[11]
- The 2-year EFS rate was 78% for patients older than 3 years, which was considerably better than the EFS rate of 11% for patients younger than 3 years.
- All but one of the surviving patients (seven of eight) in the older group received craniospinal irradiation and intensive chemotherapy with hematopoietic stem cell transplant.
- Only 3 of the 22 younger patients received any form of radiation therapy, 2 of whom were disease free.
- In a Surveillance, Epidemiology, and End Results (SEER) Program registry review, radiation therapy was associated with improved survival in children younger than 3 years.[12]
- In the European Registry for rhabdoid tumors series, the following results were observed:[13][Level of evidence C1]
- Radiation therapy was also associated with improved survival, with a 6-year OS rate of 66% (± 0.1%) in patients who received this treatment.
- The significant benefit of radiation therapy was corroborated in an extension of this series.[14]
Evidence (multimodality therapy):
- The IRS-III study used radiation therapy, intrathecal methotrexate, cytarabine, hydrocortisone, and systemic multiagent chemotherapy. The results of this small retrospective series were encouraging,[15,16] leading to the first prospective study of multimodality treatment in this group of patients.
- On the basis of the previous pilot series, a prospective multi-institutional trial was conducted for children with newly diagnosed CNS AT/RT. Treatment was divided into five phases: preirradiation, chemoradiation, consolidation, maintenance, and continuation therapy. Intrathecal chemotherapy was administered, alternating intralumbar and intraventricular routes. Radiation therapy was either focal (54 Gy) or craniospinal (36 Gy, plus primary boost), depending on the child's age and extent of disease at diagnosis.[7]
- The 2-year PFS rate was 53% (± 13%), and the 2-year OS rate was 70% (± 10%).
- Results were most favorable for children who were older, had a gross-total resection, and had no metastatic disease at presentation.
- Six of the eight children without progressive disease at the time of the report had received conformal radiation therapy, and two children had received craniospinal radiation therapy. Seven children had a gross-total resection, and only one child had metastatic disease (this child had persistent, stable disease 1.5 years from diagnosis).
- The COG performed a prospective single-arm study of 65 children. Fifty-four of the children were younger than 36 months and received two courses of methotrexate, cyclophosphamide, cisplatin, and etoposide followed by three courses of high-dose carboplatin and thiotepa supported by peripheral stem cell rescue. For patients with nondisseminated disease, focal involved-field radiation therapy was mandated after either induction or consolidation, depending on age. For patients with disseminated disease, craniospinal radiation at the end of therapy was recommended but not mandated.[8]
- For all patients, the 4-year EFS rate was 37%, and the 4-year OS rate was 43%.
- For children younger than 36 months at diagnosis, the 4-year EFS rate was 35%, compared with 6.4% in a historical cohort of patients who received chemotherapy alone (P < .0005).
- For the 11 children aged 36 months or older at diagnosis, the 4-year EFS rate was 48%, and the 4-year OS rate was 57%.
- Toxicity from this regimen was significant. Four treatment-related deaths (6% of the patients) resulting from sepsis, respiratory failure, or CNS necrosis were reported.
On the basis of the two prospective studies summarized above, multimodality therapy with surgery, radiation therapy, and chemotherapy seems to be the best treatment to optimize the survival of children with AT/RT. However, toxicities can be significant, and the most effective regimen and the optimal sequencing of therapies still need to be determined.
Treatment Options Under Clinical Evaluation
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the Children's Oncology Group, the Pediatric Brain Tumor Consortium, or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
References:
- Hilden JM, Meerbaum S, Burger P, et al.: Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol 22 (14): 2877-84, 2004.
- Packer RJ, Biegel JA, Blaney S, et al.: Atypical teratoid/rhabdoid tumor of the central nervous system: report on workshop. J Pediatr Hematol Oncol 24 (5): 337-42, 2002 Jun-Jul.
- Geyer JR, Sposto R, Jennings M, et al.: Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol 23 (30): 7621-31, 2005.
- Nicolaides T, Tihan T, Horn B, et al.: High-dose chemotherapy and autologous stem cell rescue for atypical teratoid/rhabdoid tumor of the central nervous system. J Neurooncol 98 (1): 117-23, 2010.
- Gardner SL, Asgharzadeh S, Green A, et al.: Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer 51 (2): 235-40, 2008.
- Finkelstein-Shechter T, Gassas A, Mabbott D, et al.: Atypical teratoid or rhabdoid tumors: improved outcome with high-dose chemotherapy. J Pediatr Hematol Oncol 32 (5): e182-6, 2010.
- Chi SN, Zimmerman MA, Yao X, et al.: Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 27 (3): 385-9, 2009.
- Reddy AT, Strother DR, Judkins AR, et al.: Efficacy of High-Dose Chemotherapy and Three-Dimensional Conformal Radiation for Atypical Teratoid/Rhabdoid Tumor: A Report From the Children's Oncology Group Trial ACNS0333. J Clin Oncol 38 (11): 1175-1185, 2020.
- Zaky W, Dhall G, Ji L, et al.: Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer 61 (1): 95-101, 2014.
- Aridgides PD, Mahajan A, Eaton B, et al.: Focal versus craniospinal radiation for disseminated atypical teratoid/rhabdoid tumor following favorable response to systemic therapy. Pediatr Blood Cancer 70 (7): e30351, 2023.
- Tekautz TM, Fuller CE, Blaney S, et al.: Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol 23 (7): 1491-9, 2005.
- Buscariollo DL, Park HS, Roberts KB, et al.: Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a Surveillance, Epidemiology, and End Results analysis. Cancer 118 (17): 4212-9, 2012.
- Bartelheim K, Nemes K, Seeringer A, et al.: Improved 6-year overall survival in AT/RT - results of the registry study Rhabdoid 2007. Cancer Med 5 (8): 1765-75, 2016.
- Frühwald MC, Hasselblatt M, Nemes K, et al.: Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors. Neuro Oncol 22 (7): 1006-1017, 2020.
- Olson TA, Bayar E, Kosnik E, et al.: Successful treatment of disseminated central nervous system malignant rhabdoid tumor. J Pediatr Hematol Oncol 17 (1): 71-5, 1995.
- Zimmerman MA, Goumnerova LC, Proctor M, et al.: Continuous remission of newly diagnosed and relapsed central nervous system atypical teratoid/rhabdoid tumor. J Neurooncol 72 (1): 77-84, 2005.
Treatment of Recurrent Childhood CNS Atypical Teratoid / Rhabdoid Tumor
There is no standard treatment for children with recurrent central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
Trials of molecularly targeted therapy are ongoing. In a study of the EZH2 inhibitor tazemetostat in adult patients with epithelioid sarcoma and non-CNS malignant rhabdoid tumors with SMARCB1 or SMARCA4 loss, prolonged stable disease and objective responses were observed.[1] In the National Cancer Institute (NCI)–Children's Oncology Group Pediatric MATCH APEC1621C (NCT03213665) trial, eight children with AT/RT received tazemetostat. One patient demonstrated disease stabilization.[2][Level of evidence B4]
Stereotactic radiation therapy/radiosurgery or focal radiation therapy can also be considered for the treatment of children with recurrent disease.[3]
Patients or families who desire additional disease-directed therapy should consider entering trials of novel therapeutic approaches because no standard agents have demonstrated clinically significant activity.
Regardless of whether a decision is made to pursue disease-directed therapy at the time of progression, palliative care remains a central focus of management. This ensures that quality of life is maximized while attempting to reduce symptoms and stress related to the terminal illness.
Treatment Options Under Clinical Evaluation
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the Children's Oncology Group (COG), the Pediatric Brain Tumor Consortium, or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- PEPN2121 (NCT05286801) (Tiragolumab and Atezolizumab for the Treatment of Relapsed or Refractory SMARCB1- or SMARCA4-Deficient Tumors): This study is evaluating the combination of a PD-L1 targeting antibody (atezolizumab) with a TIGIT targeting antibody (tiragolumab) for patients with SMARCB1- or SMARCA4-deficient tumors. Patients with AT/RT may be eligible for this study.
References:
- Italiano A, Soria JC, Toulmonde M, et al.: Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19 (5): 649-659, 2018.
- Chi SN, Yi JS, Williams PM, et al.: Tazemetostat for tumors harboring SMARCB1/SMARCA4 or EZH2 alterations: results from NCI-COG pediatric MATCH APEC1621C. J Natl Cancer Inst 115 (11): 1355-1363, 2023.
- Spina A, Gagliardi F, Boari N, et al.: Does Stereotactic Radiosurgery Positively Impact the Local Control of Atypical Teratoid Rhabdoid Tumors? World Neurosurg 104: 612-618, 2017.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Latest Updates to This Summary (04 / 05 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
This summary was comprehensively reviewed.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood central nervous system atypical teratoid and rhabdoid tumor. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment are:
- Kenneth J. Cohen, MD, MBA (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital)
- Karen J. Marcus, MD, FACR (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Roger J. Packer, MD (Children's National Hospital)
- D. Williams Parsons, MD, PhD (Texas Children's Hospital)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/hp/child-cns-atrt-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389426]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2024-04-05
This information does not replace the advice of a doctor. Healthwise, Incorporated disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content.
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Healthwise, Incorporated.


